ni 2+ electron configuration|electron configuration chart : Cebu Electron configuration for Ni is #1s^2 2s^2 2p^6 4s^2 3d^8#. #Ni^(2+)# has two electrons less than Ni ( that is why #Ni^(2+)# is positively charged). So when writing electron configuration for #Ni^(2+)# we exclude last two electrones from the last shell of Ni electron confgiruation. 14-day weather forecast for Maidstone.
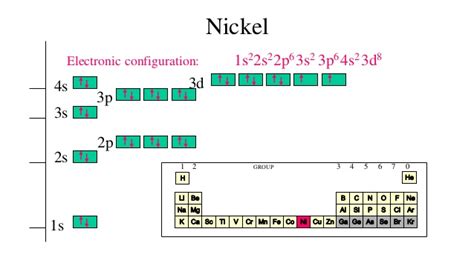
ni 2+ electron configuration,Nickel ion(Ni 2+, Ni 3+) electron configuration. The electron configuration of nickel shows that the last shell of nickel has two electrons and the d-orbital has a total of eight electrons. Therefore, the valence electrons of nickel are ten. There are two types of .Either way, the Nickel electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d8 4s2 Note that when writing the electron configuration for an atom like Ni, the 3d is usually written before the.
electron configuration chartElectron configuration for Ni is #1s^2 2s^2 2p^6 4s^2 3d^8#. #Ni^(2+)# has two electrons less than Ni ( that is why #Ni^(2+)# is positively charged). So when writing electron configuration for #Ni^(2+)# we exclude last two electrones from the last shell of Ni electron confgiruation.

In the ground state, the electron configuration of the transition metals follows the format, ns 2 nd x. As for the electron configuration for transition metals that are charged (i.e. Cu + ), the electrons from the s orbital will be moved .
Ni2+ electron configuration. As discussed, earlier Ni2+ belongs to the 3d block element so it has 8 d electrons in the outermost shell. The total number of electrons in Ni2+ is 26. They are distributed as follows, 1s22s22p63s23p63d84s2. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Nickel (Ni) .ni 2+ electron configuration electron configuration chart The number of unpaired electrons for Ni 2+ is two. From the box diagram and Ni2+ electron configuration, it is evident that among 8 electrons of Ni 2+ in d orbital, six electrons are paired in d xy, d yz, d zx, orbitals, and two electrons remain in unpaired form in d x 2 – y 2 and d z 2 orbital. So, the number of unpaired electrons in Ni 2 . For example, silicon has nine possible integer oxidation states from −4 to +4, but only -4, 0 and +4 are common oxidation states. Nickel - Electron Configuration and Oxidation States - Ni. Electron configuration of Nickel is [Ar] 3d8 4s2. Possible oxidation states are +2,3.The ground-state electron configuration of a Ni 2+ ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 . Therefore, Ni 2+ is. paramagnetic with two unpaired electrons. diamagnetic. paramagnetic with one unpaired electron. paramagnetic with four unpaired electrons. paramagnetic with five unpaired electrons. Answer.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).The periodic table gives the following electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 3. The reason why this electron configuration seems more complex is that the f-block, the Lanthanide series, is involved. Most students who first learn electron configurations often have trouble with .In [Ni(CN) 4] 2-, there is Ni 2+ ion for which the electronic configuration in the valence shell is 3d 8 4s 0. In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp 2 hybridization to make bonds with CN- ligands in square planar geometry. Thus [Ni(CN) 4] 2- is diamagnetic.The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 2.6.2 2.6. 2 ): A superscript number that designates the number of electrons in that particular subshell.ni 2+ electron configuration Atomic Number of Nickel (Ni) = 28 Electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [Ar], 3d8, 4s2 where [Ar] is Argon with atomic number 18 Ni 2 +: [Ar] 4s 0 3d 8. Or simply Ni 2 +: [Ar] 3d 8. In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest energy level of n = 4 in this case. Therefore, the s-orbital will lose its electrons first, before the d-orbital, and so Ni 2+ can be written as .In this case, the nickel atom carries a positive charge. Ni – 2e – → Ni 2+. Here, the electron configuration of nickel ion (Ni 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8. This nickel ion (Ni 2+) has twenty-eight protons, thirty-one . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 58 Ni Electron configuration [Ar] 3d 8 4s 2 CAS number: 7440-02-0 ChemSpider ID: 910: ChemSpider is a free . The electronic configuration is defined as the number of electrons distributed in the atom’s or molecule’s orbits. Nickel has 28 electrons which are distributed among the 4 orbits of its atom. Electronic configuration of nickel can be written as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2 or it can also be written as [Ar] 3d 8 4s 2. The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.Write the long and short electron configuration for {eq}Ni^{2+} {/eq}. Nickel. Nickel is an element with the symbol 'Ni' having atomic number 28 and atomic mass 58.6934 u. It is a silvery-white metal with a slight golden shade. It is hard and ductile. Nickel belongs to the transition metal d block elements in the periodic table.
From Sc on, the 3 d orbitals are actually lower in energy than the 4 s orbital, which means that electrons enter the 3 d orbitals first. In this video, we’ll discuss this in more depth and .

The Nickel electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 and its atomic number is 28. So there are 28 electrons and protons present in it. . The steps for writing the electron configuration of Ni have been discussed below: The criteria for writing electron configuration is obeying the three rules: Aufbau Principle, .
ni 2+ electron configuration|electron configuration chart
PH0 · how to write electron configuration
PH1 · electron configuration worksheet
PH2 · electron configuration of v3+ ion
PH3 · electron configuration chart
PH4 · Iba pa